Buffer Definition Biology Example
In nature there are many systems that use buffering for pH regulation. You can explore more about buffer solutions here.
 Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
A classic example of a weak acid based buffer is acetic acid CH3COOH and sodium acetate CH3COONa.

Buffer definition biology example. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. They help maintain a given pH even after the addition of an acid or a base.
Serve asact asbe a buffer Savings can be a buffer during times of financial hardship. A mixture of an acid and its conjugate base salt such as H2CO3or HCO3-. Role in pH Regulation 9.
Role of Lungs and Kidneys in pH Regulation 11. Principles of Buffers 3. Its pH changes very little when a small amount of strong acid or base is added to it.
For example the bicarbonate buffering system is used to regulate the pH of blood. For instance one of the buffers that maintain the pH of human blood involves carbonic acid H. Acidic buffers are solutions that have a pH below 7 and contain a weak acid and one of its salts.
H2PO4-HPO42- that when present in a solution reduces any changes in pH that would otherwise occur in the solution when acid or alkali is added to it. Buffers are used to make solutions of known pH especially for instrument calibration purposes. The hydrogen ion concentration of the buffer solution is given by the expression.
Definition of Buffers 2. A buffer is a chemical system designed to prevent dramatic alterations in fluid pH by binding up any changes in hydrogen ion concentrations due to excess acid or base production. 1 chemistry A buffer solution.
An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH. This solution is quite important in the field of chemistry. Most buffers consist of a weak acid and a weak base.
A buffer solution refers to an aqueous solution. Buffer Pairs in the Blood 6. In water solution sodium acetate is completely dissociated into sodium Na and acetate CH 3 COO - ions.
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Note- A lot of biological chemical reactions need a constant pH for the reaction to proceed.
Thus the pH of the blood and body fluids is kept relatively constant pH 745 although acid metabolites are continually being formed in the tissues and CO2is lost in the lungs. For example a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 475. An example of a common buffer is a solution of acetic acid CH 3 COOH and sodium acetate.
Acidosis and Alkalosis Acidosis 10. Determining the pH 4. Someone or something that provides protection against difficult situations problems angry people etc.
A solution containing either a weak acid and a conjugate base or a weak base and a conjugate acid used to stabilize the pH of a liquid upon dilution. For example blood contains a carbonic acid H 2 CO 3-bicarbonate HCO 3- buffer system. A buffer is an aqueous solution consisting of a mixture of a weak acid its salt or a weak base its salt that resist a change in pH on the addition of either acid or base.
Furthermore it consists of a mixture of a weak acid and its conjugate base or vice-versa. Chemistry A substance that prevents change in the acidity of a solution when an acid or base is added to the solution or when the solution is diluted. Renal Correction of.
A buffer against sth A special committee was formed to serve as a buffer against customer complaints. Elimination of Free Acids 12. A common weak base buffer is made of ammonia NH3 and ammonium chloride NH4Cl.
Tissue Fluids and Tissues 8. In chemistry buffer solution and examplesIt is a solution containing either a weak acid and its salt or a weak base and its salt which resists changes in pH. Renal Correction of Acidosis 13.
In this system the weak acid dissociates to a small extent giving bicarbonate ions. Alkaline buffers on the other hand have a pH above 7 and contain a weak base and one of its salts. In other words a buffer is an aqueous solution of a weak acid and its conjugate base or a weak base and its conjugate acid.
Buffers typically consist of an acid-base pair with the acid and base differing by the presence or absence of a proton a conjugate acid-base pair. 2 biochemistry An ionic compound that when added to a solution neutralizes both acids and bases without significantly changing the original acidity or alkalinity of a solution.
 Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
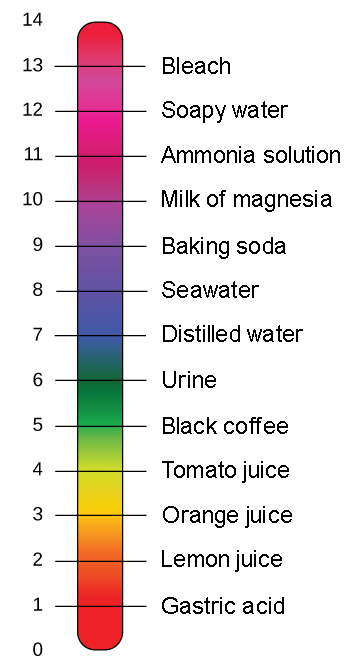 Buffers Ph Acids And Bases Biology For Non Majors I
Buffers Ph Acids And Bases Biology For Non Majors I
 Buffer Solutions Definition Types Preparation Examples And Videos
Buffer Solutions Definition Types Preparation Examples And Videos
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
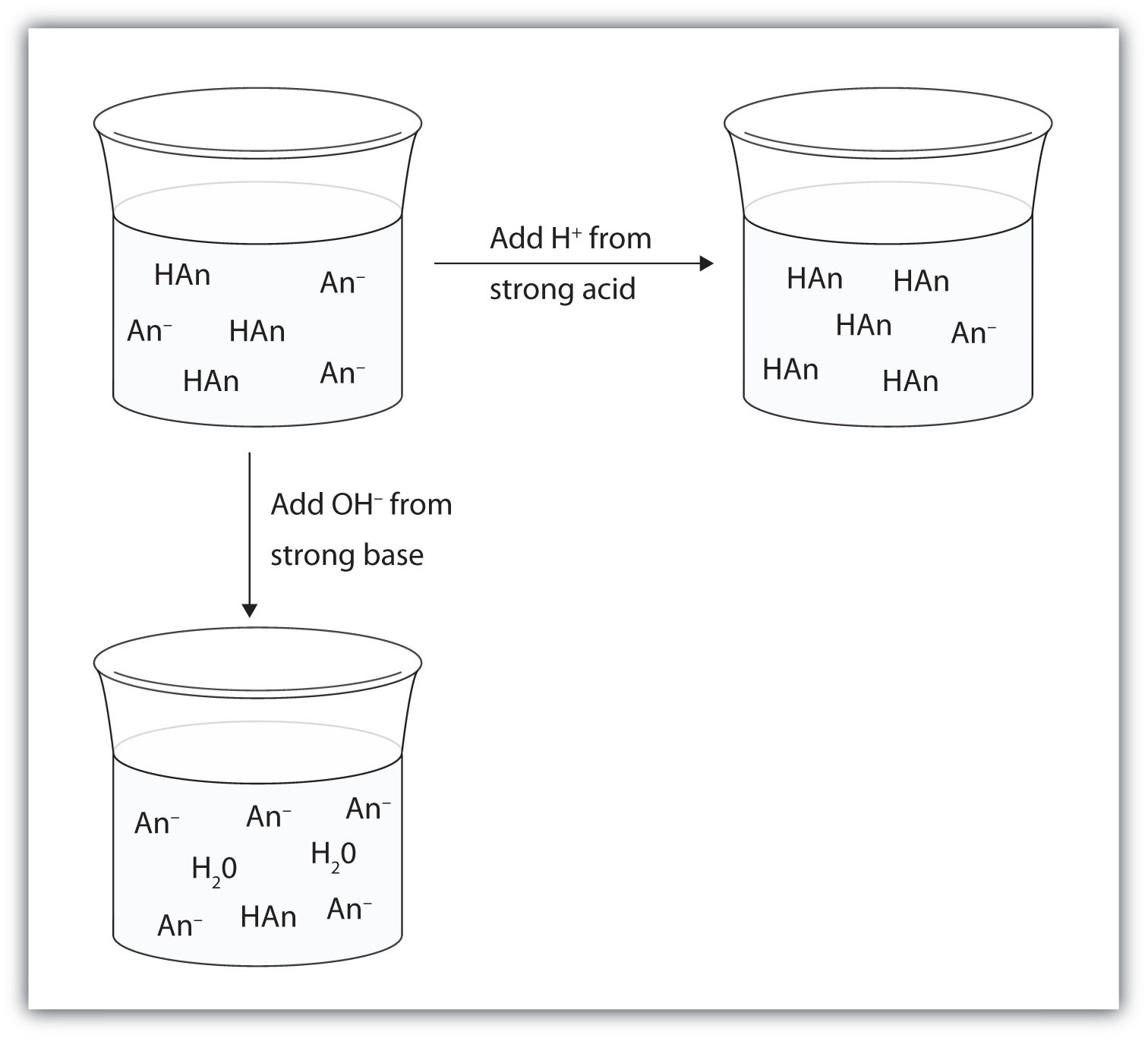 10 5 Buffers The Basics Of General Organic And Biological Chemistry
10 5 Buffers The Basics Of General Organic And Biological Chemistry
 A A Diagram Of The Process Of Agarose Gel Electrophoresis 1 An Agarose And Buffer Solution Is Heated And Poured Microbiology Study Chemistry Study Biology
A A Diagram Of The Process Of Agarose Gel Electrophoresis 1 An Agarose And Buffer Solution Is Heated And Poured Microbiology Study Chemistry Study Biology
 Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
 Recombinant Dna Definition Steps Examples Invention Recombinant Dna Dna Technology Dna
Recombinant Dna Definition Steps Examples Invention Recombinant Dna Dna Technology Dna
 Coordinate Covalent Bond Definition Examples Formation And Properties Covalent Bonding Coordinates Cool Websites
Coordinate Covalent Bond Definition Examples Formation And Properties Covalent Bonding Coordinates Cool Websites
 Types Of Hydrogen Isotopes Chemistry Basics Science Chemistry Chemistry
Types Of Hydrogen Isotopes Chemistry Basics Science Chemistry Chemistry
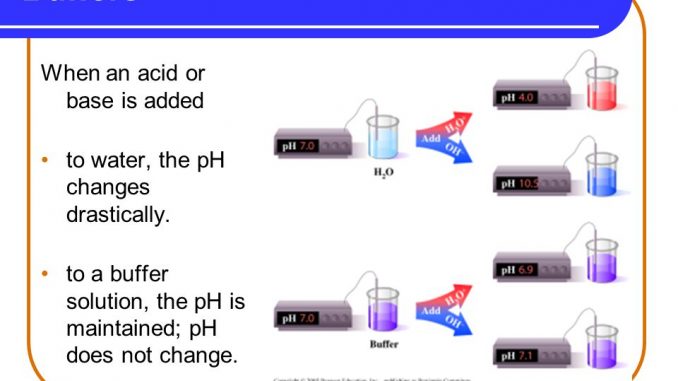 Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
 Why Isotopes Are Unstable Chemistry Basics Chemistry Science Student
Why Isotopes Are Unstable Chemistry Basics Chemistry Science Student
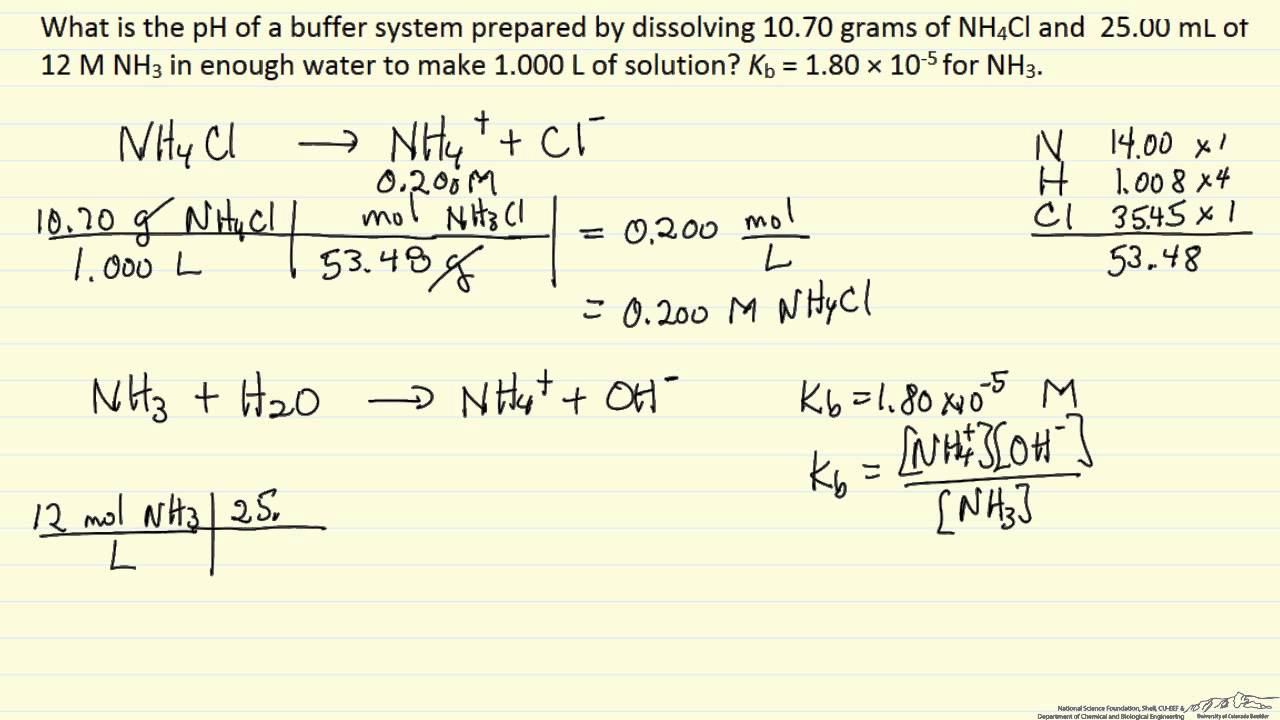 Ph Of Buffer Solution Example Youtube
Ph Of Buffer Solution Example Youtube
Ph Buffers Acids And Bases Introduction To Chemistry



